What is Atypical Mycobacteria Antibiotic Therapy (AMAT)?
 For Crohn’s disease patients who have tested positive for human paratuberculosis or another form of atypical mycobacteria, Atypical Mycobacteria Antibiotic Therapy (AMAT) may be beneficial. This therapy combines antibiotics that specifically target human paratuberculosis (MAP). It is sometimes sustained for years in an attempt to eradicate the pathogen and avoid bacterial resistance. Different combinations of antibiotics in differing strengths and duration have been tested, with generally positive results. Some of these antibiotics include clarithromycin, Rifabutin, rifampin, clofazimine, Ciprofloxacin, metronidazole and levofloxacin. For a meta-analysis of the published AMAT studies, see Primary treatment of Crohn’s disease: combined antibiotics taking center stage. The below table summarizes the results of the AMAT studies detailed in this analysis.
For Crohn’s disease patients who have tested positive for human paratuberculosis or another form of atypical mycobacteria, Atypical Mycobacteria Antibiotic Therapy (AMAT) may be beneficial. This therapy combines antibiotics that specifically target human paratuberculosis (MAP). It is sometimes sustained for years in an attempt to eradicate the pathogen and avoid bacterial resistance. Different combinations of antibiotics in differing strengths and duration have been tested, with generally positive results. Some of these antibiotics include clarithromycin, Rifabutin, rifampin, clofazimine, Ciprofloxacin, metronidazole and levofloxacin. For a meta-analysis of the published AMAT studies, see Primary treatment of Crohn’s disease: combined antibiotics taking center stage. The below table summarizes the results of the AMAT studies detailed in this analysis.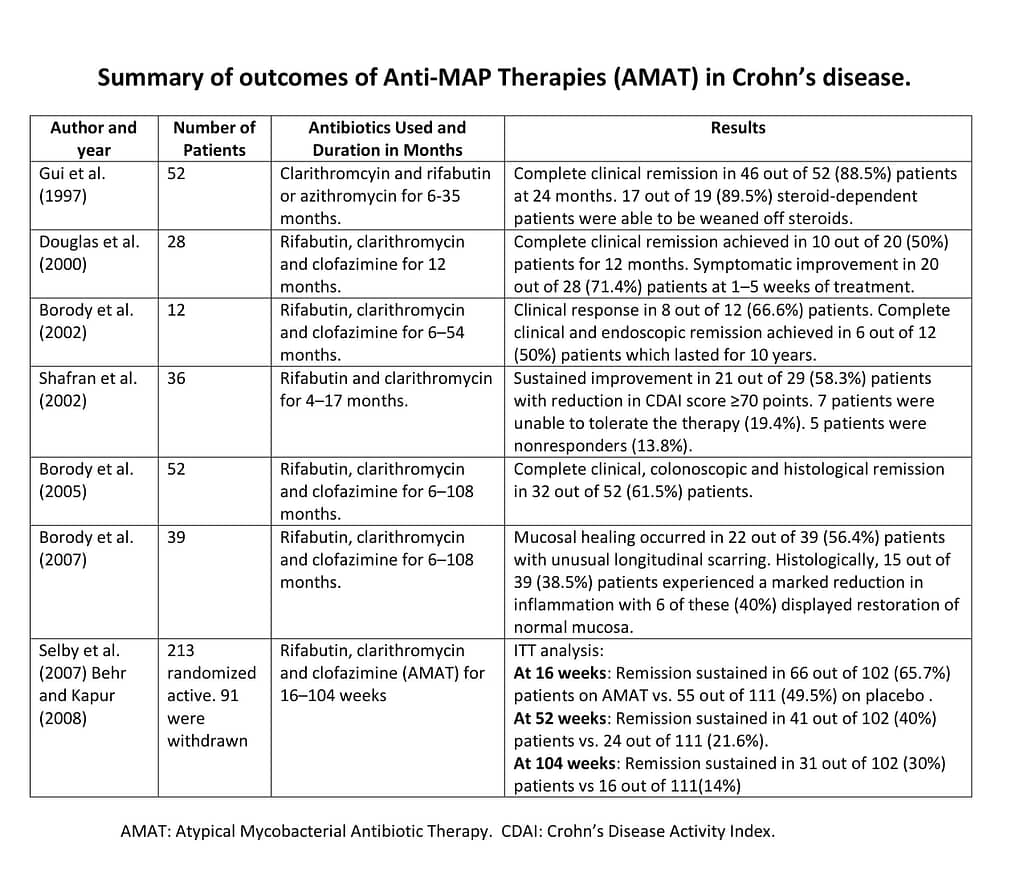 Comparing remission rates for AMAT therapy to the Anti-TNF medications, which are currently the costly gold standard of care for Crohn’s disease patients around the world, yields surprising results. The below chart shows the results of the 2007 Selby study, a large, randomized AMAT study, along with the data for the major Humira and Remicade studies. While the Selby study concluded that AMAT showed no benefit over a placebo, the analysis and results were flawed due to sub-optimal doses of antibiotics and capsules that failed to rupture. In fact, when the results were viewed in an intention-to-treat (ITT) analysis, the remission rates for AMAT were significantly better than those of the Anti-TNF therapies.
Comparing remission rates for AMAT therapy to the Anti-TNF medications, which are currently the costly gold standard of care for Crohn’s disease patients around the world, yields surprising results. The below chart shows the results of the 2007 Selby study, a large, randomized AMAT study, along with the data for the major Humira and Remicade studies. While the Selby study concluded that AMAT showed no benefit over a placebo, the analysis and results were flawed due to sub-optimal doses of antibiotics and capsules that failed to rupture. In fact, when the results were viewed in an intention-to-treat (ITT) analysis, the remission rates for AMAT were significantly better than those of the Anti-TNF therapies.
An ITT analysis includes every subject who is randomized according to randomized treatment assignment. It ignores non-compliance, protocol deviations, withdrawal, and anything that happens after randomization. ITT analysis avoids over optimistic estimates of the efficacy of a treatment resulting from the removal of non-compliers by accepting that non-compliance and protocol deviations are likely to occur in actual clinical practice. For example, in the Accent I trial which looked at the effectiveness of Remicade, there were initially 573 people who were given the first dose of Remicade. However, only the 335 people who responded favorably to that initial dose after two weeks continued in the study. An ITT analysis would include all 573, randomly assigned to three groups for a total of 191 in each arm. Looking at the results for the highest dosage arm, a total of 50 people were in remission at 30 weeks, so the final remission rate was 26% (50/191) using an ITT analysis. In the end, even a sub-par dose of AMAT was more effective than the highest doses of the Anti-TNF medications.
Links to the Selby Study, the Accent I Trial and the Charm Trial.
Bear in mind, the remission rates in these abstracts will be different than the remission rates in the table above due to an alternate analysis. Additionally, drug trials are not generally compared side by side. One of the commonly prescribed AMAT drugs, clofazimine, is no longer available in some Western countries, including the United States. This medication is primarily used to treat leprosy, uncommon in developed countries. While not banned, supply was discontinued due to non-use. More information on how to obtain clofazimine is available HERE.
Antibiotics in Pediatric IBD
One small study with 10 pediatric patients (age 8 to 17) from 2013 reported an 80% remission rate when treatment with RHB-104 was given for at least 6 months. AMAT was particularly effective in patients who had not previously taken immunosuppressive medications. A new study presented at the 2016 American College of Gastroenterology Meeting reported that 8 patients who were given AMAT as a first-line therapy achieved remission and experienced histological deep mucosal healing.
In 2019, researchers at the Children’s Hospital of Philadelphia looked at 63 pediatric patients with refractory IBD who had been treated with antibiotics and concluded that “antibiotics appears to be an effective rescue and steroid-sparing therapy to induce remission in the short term in patients failing a biologic.”
Fluoroquinoline Antibiotics and AMAT
 On July 26, 2016, the United States Food and Drug Administration (FDA) issued a warning regarding the use of antibiotics in the fluoroquinolone class. These include levofloxacin (Levaquin) and ciprofloxacin (Cipro) which are occasionally used in AMAT protocols. The FDA warns that fluroquinolones are associated with disabling side effects involving tendons, muscles, joints, nerves and the central nervous system. These side effects can occur hours to weeks after exposure to fluoroquinolones and may potentially be permanent. Therefore, the FDA has determined that fluoroquinolones should be reserved for use in patients with serious conditions who have no alternative treatment options. Due to this warning, mindful consideration should be given when including fluoroquinolones in any AMAT protocol. For more information, please see the full FDA News Release and Dr. William Chamberlin’s recent article on this topic.
On July 26, 2016, the United States Food and Drug Administration (FDA) issued a warning regarding the use of antibiotics in the fluoroquinolone class. These include levofloxacin (Levaquin) and ciprofloxacin (Cipro) which are occasionally used in AMAT protocols. The FDA warns that fluroquinolones are associated with disabling side effects involving tendons, muscles, joints, nerves and the central nervous system. These side effects can occur hours to weeks after exposure to fluoroquinolones and may potentially be permanent. Therefore, the FDA has determined that fluoroquinolones should be reserved for use in patients with serious conditions who have no alternative treatment options. Due to this warning, mindful consideration should be given when including fluoroquinolones in any AMAT protocol. For more information, please see the full FDA News Release and Dr. William Chamberlin’s recent article on this topic.
AMAT Protocols
From patients who have provided this information publicly, the most commonly used daily AMAT protocol is: 1000 mg. of clarithromycin, 300 mg. of Rifabutin, 2 mg. per kg. of clofazimine. These doses are split in half and taken throughout the day. Some doctors prefer to ramp up this therapy to minimize initial side effects, starting with lower doses and gradually increasing the strength to the full dose over time. See below for more details on the RedHill Biopharma trial utilizing a version of this protocol. Some doctors substitute 600 mg. of rifampin in place of the Rifabutin.
Dr. Thomas Borody, who runs the Centre for Digestive Diseases in Sydney, Australia has achieved an 85% remission rate (the highest in the world) prescribing antibiotic therapy for Crohn’s disease. The article Medical Pioneer from Down Under leads the World in Crohn’s Treatment by Warren Perley describes his approach and why this therapy has had continued success.
Dr. William Chamberlin also discusses some commonly prescribed AMAT combinations in this recent article.
RedHill Biopharma MAP US Phase III Trial
RedHill Biopharma recently completed a large scale, blinded, Phase III clinical trial at 92 locations in 10 countries to test the effectiveness of triple antibiotic therapy in Crohn’s disease. RHB-104 is a proprietary and potentially groundbreaking antibiotic combination therapy, in oral capsule formulation, with potential intracellular, antimycobacterial and anti-inflammatory properties. Developed by Dr. Thomas Borody, it’s based on increasing evidence supporting the hypothesis that Crohn’s disease is caused by a human MAP infection in certain susceptible patients. Study design was detailed (prior to the release of results) by Dr. Ira Kalfus at the 2017 MAP Conference at Temple University.
Results from the study were overwhelmingly successful. Top-line results demonstrated superiority of RHB-104 over placebo in achieving remission at week 26, defined as Crohn’s Disease Active Index (CDAI) value of less than 150, which was the primary endpoint of the study. The proportion of patients meeting the primary endpoint on an intent-to-treat basis was significantly greater in the RHB-104 group compared to placebo (37% vs. 23%, p= 0.0048)
Primary endpoint results were detailed in 2018 by Dr. David Graham at United European Gastroenterology Week in Vienna. Beginning on Page 23 of the RedHill June 2019 Corporate Presentation the study showed a significant decline in fecal calprotection levels by patients taking RHB-104 vs. placebo.
To learn more about the RHB-104 study, please see the above references or visit ClinicalTrials.gov. RHB-104 contains clofazimine, rifabutin and clarithromycin. RedHill gradually increases the dosage, with each capsule containing 95 mg clarithromycin, 45 mg Rifabutin, and 10 mg clofazimine. Eventually, the patient will take 5 capsules, twice a day for a total dosage of 950 mg. of clarithromycin, 450 mg. of rifabutin, and 100 mg. of clofazimine.
Why isn’t this treatment widely available?
 Currently, AMAT is not an FDA approved treatment pathway for use in Crohn’s disease. While each individual antibiotic utilized in AMAT is approved by the FDA, they are approved for conditions other than Crohn’s disease. Their use in Crohn’s disease, or other MAP driven conditions, is therefore considered “off-label.” A medication is used “off-label” when the use is not specified on the FDA approved package label. Per the FDA regulations, doctors are allowed to prescribe a medication for an “off-label” usage “when they judge that it is medically appropriate for their patient.” For patients who have tried all available approved treatments with no benefit, doctors are allowed to consider using off-label medications. The combination of Cipro (ciprofloxacin) and Flagyl (metronidazole) is commonly prescribed off-label in Crohn’s disease for abscesses and fistulas.
Currently, AMAT is not an FDA approved treatment pathway for use in Crohn’s disease. While each individual antibiotic utilized in AMAT is approved by the FDA, they are approved for conditions other than Crohn’s disease. Their use in Crohn’s disease, or other MAP driven conditions, is therefore considered “off-label.” A medication is used “off-label” when the use is not specified on the FDA approved package label. Per the FDA regulations, doctors are allowed to prescribe a medication for an “off-label” usage “when they judge that it is medically appropriate for their patient.” For patients who have tried all available approved treatments with no benefit, doctors are allowed to consider using off-label medications. The combination of Cipro (ciprofloxacin) and Flagyl (metronidazole) is commonly prescribed off-label in Crohn’s disease for abscesses and fistulas.
Despite years of published studies, most doctors are reluctant or unwilling to treat Crohn’s disease with the AMAT protocol. One reason may be that although these individual studies give a large amount of data regarding the AMAT protocol, there is no consensus as to which combination, strength or duration of antibiotics, is most effective. Additional reasons may be:
- Funding to do the studies and research necessary has been lacking.
- Difficulty in the accurate detection of human paratuberculosis (MAP) and underestimation of the sophistication of mycobacterial disease is another treatment barrier.
- Doctors may not want to treat patients without laboratory confirmation of the bacteria.
- Potential liability of prescribing a therapy that is not an officially approved treatment pathway for Crohn’s disease.
Some well meaning, but busy gastroenterologists may find it impossible to keep up on all of the latest research, and are unaware of AMAT and it’s success. In any given year, there are about 3,000 research articles published in the field of Inflammatory Bowel Disease. This does not include the cross-field articles discussing IBD concerns related to microbiology, immunology, genetics, veterinary medicine and epidemiology; all of which have significance to the field of gastroenterology. It’s impossible to keep up on them all, so reading may be directed by opinion leaders, who may have leanings toward the political arena rather than those who understand the science best. At the end of the day, it’s hard to accept any new idea as truth.
“All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as self-evident.”
Arthur Schopenhauer
Human Paratuberculosis Foundation is striving to achieve healing and a cure for human MAP related diseases, including Crohn’s disease. We eagerly await future research studies that examine the benefit of AMAT in Crohn’s disease and search for alternative Anti-MAP therapeutic options.

