What is Multiple Sclerosis?
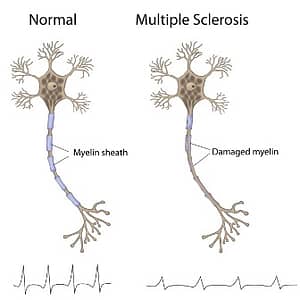 An unpredictable disease of the central nervous system, multiple sclerosis (MS) can range from relatively benign to somewhat disabling to devastating, as communication between the brain and other parts of the body is disrupted. First described in 1830, MS is a neuroinflammatory disease that affects myelin, a substance that makes up the membrane (called the myelin sheath) that wraps around nerve fibers (axons). Myelinated axons are commonly called white matter. Researchers have learned that MS also damages the nerve cell bodies, which are found in the brain’s gray matter, as well as the axons themselves in the brain, spinal cord, and optic nerve (the nerve that transmits visual information from the eye to the brain). As the disease progresses, the brain’s cortex shrinks (cortical atrophy).
An unpredictable disease of the central nervous system, multiple sclerosis (MS) can range from relatively benign to somewhat disabling to devastating, as communication between the brain and other parts of the body is disrupted. First described in 1830, MS is a neuroinflammatory disease that affects myelin, a substance that makes up the membrane (called the myelin sheath) that wraps around nerve fibers (axons). Myelinated axons are commonly called white matter. Researchers have learned that MS also damages the nerve cell bodies, which are found in the brain’s gray matter, as well as the axons themselves in the brain, spinal cord, and optic nerve (the nerve that transmits visual information from the eye to the brain). As the disease progresses, the brain’s cortex shrinks (cortical atrophy).
The term multiple sclerosis refers to the distinctive areas of scar tissue (sclerosis or plaques) that are visible in the white matter of people who have MS. Plaques can be as small as a pinhead or as large as the size of a golf ball. Doctors can see these areas by examining the brain and spinal cord using a type of brain scan called magnetic resonance imaging(MRI). While MS can cause severe disability, it is rarely fatal.
Cause of Multiple Sclerosis
The cause of multiple sclerosis is currently unknown. There are approximately 2.5 million people affected by MS world-wide, and MS is twice as common in women than in men. It is more common in colder climates and in people with northern European descent, regardless of where they live. Smoking and exposure to the Epstein-Barr virus are other risk factors. People who have high Vitamin D levels or spend more time in the sun have a lower risk of developing MS. The rate of disease has steadily increased during the twentieth century.
The ultimate result of MS is damage to myelin, nerve fibers, and neurons in the brain and spinal cord, which together make up the central nervous system (CNS). But how that happens, and why, are questions that challenge researchers. Evidence appears to show that MS is a disease caused by genetic vulnerabilities combined with environmental factors, such as infectious agents.
Although there is little doubt that the immune system contributes to the brain and spinal cord tissue destruction of MS, the exact target of the immune system attacks and which immune system cells cause the destruction isn’t fully understood. Researchers have several possible explanations for what might be going on. The immune system could be:
- fighting some kind of infectious agent that has components which mimic components of the brain.
- destroying brain cells because they are unhealthy.
- mistakenly identifying normal brain cells as foreign.
 The last possibility has been the favored explanation for many years. Research now suggests that the first two activities might also play a role in the development of MS. There is a special barrier, called the blood-brain barrier, which separates the brain and spinal cord from the immune system. If there is a break in the barrier, it exposes the brain to the immune system for the first time. When this happens, the immune system may misinterpret the brain as “foreign.” T-cells, a type of white blood cells, gain access to the brain through breaks in the blood-brain barrier and recognize myelin as foreign, causing an inflammatory process.
The last possibility has been the favored explanation for many years. Research now suggests that the first two activities might also play a role in the development of MS. There is a special barrier, called the blood-brain barrier, which separates the brain and spinal cord from the immune system. If there is a break in the barrier, it exposes the brain to the immune system for the first time. When this happens, the immune system may misinterpret the brain as “foreign.” T-cells, a type of white blood cells, gain access to the brain through breaks in the blood-brain barrier and recognize myelin as foreign, causing an inflammatory process.
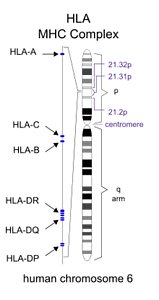
Current research suggests that dozens of genes and possibly hundreds of variations in the genetic code combine to create vulnerability to MS. Some of these genes have been identified. Most of the genes identified so far are associated with functions of the immune system. The most common mutations associated with MS are in the Human Leukocyte Antigen (HLA) complex. The HLA complex helps the immune system distinguish the body’s own proteins from proteins made by foreign invaders (such as viruses and bacteria). Changes in the HLA region have also been associated with Type 1 Diabetes and lupus.
Symptoms of Multiple Sclerosis
 Most people experience their first symptoms of MS between the ages of 20 and 40. The initial symptom of MS is often blurred or double vision, red-green color distortion, or even blindness in one eye. Many MS patients experience muscle weakness in their extremities and difficulty with coordination and balance. These symptoms may be severe enough to impair walking or even standing. In the worst cases, MS can produce partial or complete paralysis. Most people with MS also exhibit paresthesias, transitory abnormal sensory feelings such as numbness, prickling, or “pins and needles” sensations. Some may also experience pain. Speech impediments, tremors, and dizziness are other frequent complaints. Occasionally, people with MS have hearing loss. Approximately half of all people with MS experience cognitive impairments such as difficulties with concentration, attention, memory, and poor judgment, but such symptoms are usually mild and are frequently overlooked. Depression is another common feature of MS. MS often takes several forms:
Most people experience their first symptoms of MS between the ages of 20 and 40. The initial symptom of MS is often blurred or double vision, red-green color distortion, or even blindness in one eye. Many MS patients experience muscle weakness in their extremities and difficulty with coordination and balance. These symptoms may be severe enough to impair walking or even standing. In the worst cases, MS can produce partial or complete paralysis. Most people with MS also exhibit paresthesias, transitory abnormal sensory feelings such as numbness, prickling, or “pins and needles” sensations. Some may also experience pain. Speech impediments, tremors, and dizziness are other frequent complaints. Occasionally, people with MS have hearing loss. Approximately half of all people with MS experience cognitive impairments such as difficulties with concentration, attention, memory, and poor judgment, but such symptoms are usually mild and are frequently overlooked. Depression is another common feature of MS. MS often takes several forms:
 Relapsing-remitting – The most common form, 80-90% of patients experience clearly defined flares of disease interspersed with periods of partial or complete remission.
Relapsing-remitting – The most common form, 80-90% of patients experience clearly defined flares of disease interspersed with periods of partial or complete remission.
- Primary-progressive – As the name suggests, patients with this less common form experience slowly worsening symptoms from the time of disease onset without periods of flares or remission. The onset of this form is commonly later in life.
- Secondary-progressive – Patients may initially experience flares followed by periods of recovery, but the disease never disappears, and the initial period of fluctuation is followed by steadily worsening disease.
- Progressive-relapsing – In this most rare type, patient experience steadily worsening disease punctuated by acute flares seen in the relapsing-remitting form. In this form, there is continuous worsening of the disease.
Treatment of Multiple Sclerosis
 There is currently no cure for MS. Treatments aim to prevent new attacks, return function after an attack and avoid disability. Many patients do well with no therapy at all, especially since many medications have serious side effects and some carry significant risks. There are multiple medications approved for relapsing-remitting MS, but no treatment has been shown effective in primary-progressive MS, and only one therapy has been approved for secondary-progressive MS.
There is currently no cure for MS. Treatments aim to prevent new attacks, return function after an attack and avoid disability. Many patients do well with no therapy at all, especially since many medications have serious side effects and some carry significant risks. There are multiple medications approved for relapsing-remitting MS, but no treatment has been shown effective in primary-progressive MS, and only one therapy has been approved for secondary-progressive MS.
During severe flares, corticosteroids like prednisone are used, though these are a short term solution which do not appear to have a beneficial long term effect. Plasma exchange, where the plasma component of the patient’s blood is removed and reintroduced with a protein solution, is also used to treat flares.
Beta interferons, such as Avonex, Betaseron, Actoferon, Plegridy, Rebif and some biosimilars, have been approved by the Food and Drug Administration (FDA) for treatment of relapsing-remitting MS. Beta interferon has been shown to reduce the number of exacerbations and may slow the progression of physical disability. When attacks do occur, they tend to be shorter and less severe. Liver damage is a side effect of beta interferons, so liver function should be monitored during treatment. Some patients develop antibodies to beta interferons, which can reduce effectiveness.
The FDA also has approved a synthetic form of myelin basic protein, called copolymer I (Copaxone), for the treatment of relapsing-remitting MS. Copolymer I has few side effects, and studies indicate that the agent can reduce the relapse rate by almost one third. This medication must be injected, and skin irritation may occur at the injection site.
 Other FDA approved drugs to treat relapsing forms of MS in adults include teriflunomide (Aubagio), fingolimod (Gilenya), alemtuzumab (Lemtrada) and dimethyl fumarate (Tecfidera). These seek to reduce relapse rate. An immunosuppressant treatment, Novantrone (mitoxantrone), is approved by the FDA for the treatment of advanced or chronic MS. The FDA has also approved dalfampridine (Ampyra) to improve walking in individuals with MS.
Other FDA approved drugs to treat relapsing forms of MS in adults include teriflunomide (Aubagio), fingolimod (Gilenya), alemtuzumab (Lemtrada) and dimethyl fumarate (Tecfidera). These seek to reduce relapse rate. An immunosuppressant treatment, Novantrone (mitoxantrone), is approved by the FDA for the treatment of advanced or chronic MS. The FDA has also approved dalfampridine (Ampyra) to improve walking in individuals with MS.
One monoclonal antibody, natalizumab (Tysabri), was shown in clinical trials to significantly reduce the frequency of attacks in people with relapsing forms of MS and was approved for marketing by the U.S. Food and Drug Administration (FDA) in 2004. However, in 2005 the drug’s manufacturer voluntarily suspended marketing of the drug after several reports of significant adverse events. In 2006, the FDA again approved sale of the drug for MS but under strict treatment guidelines involving infusion centers where patients can be monitored by specially trained physicians.
Spasticity, which can occur either as a sustained stiffness caused by increased muscle tone or as spasms that come and go, is usually treated with muscle relaxants and tranquilizers such as baclofen, tizanidine, diazepam, clonazepam, and dantrolene. Physical therapy and exercise can help preserve remaining function. Avoiding excessive activity and avoiding heat are probably the most important measures patients can take to counter physiological fatigue. If psychological symptoms of fatigue such as depression or apathy are evident, antidepressant medications may help. Some patients use alternative or complementary therapies to deal with their MS symptoms.
RedHill Biopharma Trial of RHB-104 in Multiple Sclerosis Patients
 One emerging therapy, RHB-104, has recently completed a Stage IIa trial. The hypothesis is that MAP positive MS patients would have a greater response to interferon beta therapy plus RHB-104 than from treatment with interferon beta alone. The CEASE-MS study, which was begun in 2013 and completed in 2016 in Israel, recently released positive final results. Originally trialed in Crohn’s disease, it is showing promise in MS patients as well. Learn more about this therapy on the MAP and MS page.
One emerging therapy, RHB-104, has recently completed a Stage IIa trial. The hypothesis is that MAP positive MS patients would have a greater response to interferon beta therapy plus RHB-104 than from treatment with interferon beta alone. The CEASE-MS study, which was begun in 2013 and completed in 2016 in Israel, recently released positive final results. Originally trialed in Crohn’s disease, it is showing promise in MS patients as well. Learn more about this therapy on the MAP and MS page.
