HUMIRA (adalimumab) for Crohn’s Disease
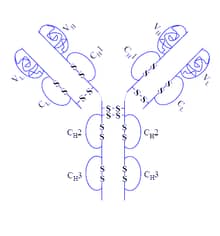 HUMIRA (generic name adalimumab), is a biologic medication approved for the treatment of Crohn’s disease. It is used in moderate to severe cases of Crohn’s disease for adult and pediatric patients over 6 years old. The name, HUMIRA, stands for HUman Monoclonal antibodyIn Rheumatoid Arthritis. Originally manufactured by BASF Bioresearch Corporation and Cambridge Antibody Technology, it was then further developed and marketed by Abbott Laboratories, which has now divided into Abbot and AbbVie, with AbbVie taking responsibility for HUMIRA. HUMIRA was the first fully human monoclonal antibody medication approved by the FDA. It was originally approved in 2002 for Rheumatoid Arthritis, but has since been approved to treat Crohn’s disease as of 2007. In addition to Crohn’s disease and Rheumatoid Arthritis, HUMIRA is also approved to treat ulcerative colitis, juvenile ideopathic arthritis, psoriatic arthritis, ulcerative colitis, severe psoriasis, ankylosing spondylitis and hidradenitis supperativa.
HUMIRA (generic name adalimumab), is a biologic medication approved for the treatment of Crohn’s disease. It is used in moderate to severe cases of Crohn’s disease for adult and pediatric patients over 6 years old. The name, HUMIRA, stands for HUman Monoclonal antibodyIn Rheumatoid Arthritis. Originally manufactured by BASF Bioresearch Corporation and Cambridge Antibody Technology, it was then further developed and marketed by Abbott Laboratories, which has now divided into Abbot and AbbVie, with AbbVie taking responsibility for HUMIRA. HUMIRA was the first fully human monoclonal antibody medication approved by the FDA. It was originally approved in 2002 for Rheumatoid Arthritis, but has since been approved to treat Crohn’s disease as of 2007. In addition to Crohn’s disease and Rheumatoid Arthritis, HUMIRA is also approved to treat ulcerative colitis, juvenile ideopathic arthritis, psoriatic arthritis, ulcerative colitis, severe psoriasis, ankylosing spondylitis and hidradenitis supperativa.
Administered as a subcutaneous injection, patients are taught the proper method of injection by their health care provider, and then have the option of injecting themselves at home. The prescribed dose of HUMIRA can be delivered to the patient directly in either a preloaded syringe or a pen injection device. This can make HUMIRA a more accessible option than the biologic treatments which require dosage at an infusion center.
When first beginning treatment, patients are traditionally given an initial dose of 160 mg. administered in four separate 40 mg. injections. These injections can either be given in one day, or split over two days. The initial dose is followed by an 80 mg. dose 2 weeks later (two injections of 40 mg. each) and finally the maintenance dose of 40 mg. (one injection) is begun 4 weeks after the first. This ramp up is necessary to establish a clinical level of the drug, after which many patients will receive a 40 mg. dose (one injection) every 2 weeks. The standard pediatric dosage, for children under 88 lbs, is half of the adult dosage. Some patients may require a 40 mg. dose every week, instead of the every two weeks, to benefit from HUMIRA. A PPD skin test for tuberculosis must be performed prior to starting treatment because HUMIRA can reactivate tuberculosis in people that have a history of the disease.
How Does HUMIRA Work?
HUMIRA is classified as a fully human monoclonal antibody that works by binding to tumor necrosis factor alpha (TNF-α). To break that down into common terms, a monoclonal antibody is a laboratory made protein which is cloned from a single unique parent cell so that it is specific to one target in the body. Similar to a lock and key mechanism, each monoclonal antibody will only bind to one substance (antigen). In this case, that substance is TNF-α. HUMIRA is a fully human monoclonal antibody, meaning that it is produced using only human DNA sources. When monoclonal antibodies are produced from animal sources, there can be problems of rejection and host reactions. This is minimized by building using fully human DNA sources to build the antibody.
HUMIRA works by binding to TNF-α and blocking it from binding to the corresponding TNF-α receptors, which then produces an inflammatory response. To fully understand how HUMIRA works, we must discuss why TNF-α is important in the Crohn’s disease process. TNF-α is a protein (cytokine) that is primarily produced by the macrophages as a part of the body’s immune and inflammation response. Macrophages are white blood cells that roam around the body looking for foreign substances to devour. They are a key component of innate immunity, and their response is believed to be dysfunctional in Crohn’s disease. The immune system and the inflammatory response is very complex, but for our purposes, it is enough to know that the macrophages produce the chemical TNF-α either in a defective way or in response to a foreign invader (like a mycobacteria) that they cannot fully recognize. Once TNF-α is released, it begins a chain of inflammatory pathways. It is this inflammatory response that causes the damage in Crohn’s disease. HUMIRA, by interrupting the action of TNF-α, halts the inflammatory process that leads to disease.
 While HUMIRA binds only to TNF-α specifically, it does so throughout the entire body. Therefore, while the benefit of HUMIRA relieves the symptoms of Crohn’s disease, it can also impair the immune system from responding to other, unrelated threats. Therefore, infections unrelated to Crohn’s disease can present a much more serious problem for patients when on HUMIRA. It is for this reason that a test for latent tuberculosis should be performed on each patient prior to beginning treatment with HUMIRA. In people who have been sick with tuberculosis and then recovered, the disease is contained and walled off in their lungs by the immune system, which forms a type of “jail” around any live bacteria. As long as the immune system remains fully functional, this jail stays intact and the contained tuberculosis cannot cause active disease. But when the patient’s immune system is impaired, either by medications, disease or lifestyle changes such as stress or depression, tuberculosis can escape its containment and cause disease. Therefore, HUMIRA should be used with extreme caution in patients who have had tuberculosis. Additionally, patients on HUMIRA should not receive live vaccines.
While HUMIRA binds only to TNF-α specifically, it does so throughout the entire body. Therefore, while the benefit of HUMIRA relieves the symptoms of Crohn’s disease, it can also impair the immune system from responding to other, unrelated threats. Therefore, infections unrelated to Crohn’s disease can present a much more serious problem for patients when on HUMIRA. It is for this reason that a test for latent tuberculosis should be performed on each patient prior to beginning treatment with HUMIRA. In people who have been sick with tuberculosis and then recovered, the disease is contained and walled off in their lungs by the immune system, which forms a type of “jail” around any live bacteria. As long as the immune system remains fully functional, this jail stays intact and the contained tuberculosis cannot cause active disease. But when the patient’s immune system is impaired, either by medications, disease or lifestyle changes such as stress or depression, tuberculosis can escape its containment and cause disease. Therefore, HUMIRA should be used with extreme caution in patients who have had tuberculosis. Additionally, patients on HUMIRA should not receive live vaccines.
Side Effects of HUMIRA
On April 14, 2016, The United States Food and Drug Administration (FDA) issued a Safety Communication concerning TNF-alpha blockers (like Remicade and HUMIRA), Azathioprine and 6-MP. This goal of the alert was to inform patients who were taking these medications that they could be at an increased risk for developing a rare cancer (Hepatosplenic T-Cell Lymphoma or HSTCL) which often results in death. A table with detailed case reports broken down by each drug is included in the alert. Through December 31, 2010, there were 2 reported cases of HSTCL in patients taking HUMIRA for rheumatoid arthritis, and 5 cases in patients taking both Remicade and HUMIRA. However, HUMIRA was not approved for Crohn’s disease until 2007 and usage has significantly increased since 2010. Updated data has been requested. To read the full alert, see the FDA Safety Communication. Patients who are concerned about their risk of HSTCL should consult their health care provider.
Most common side effects of HUMIRA include:
- Injection site reactions, such as redness, pain or swelling
- Increased likelihood of upper respiratory infections
- Rash and fever
- Headache
Serious, but rare, side effects of HUMIRA include:
- Serious infections, including tuberculosis, which spread through the body sometimes resulting in death.
- Fungal infections, including reactivation of histoplasmosis.
- Hepatitis B in those who carry the virus.
- Severe allergic reaction
- Nervous system problems
- Blood problems
- Heart failure
- Lupus-like syndrome
- Psoriasis
- Cancer: including a rare type of cancer called hepatosplenic T-cell lymphoma which often results in death. There is an increased risk for cancers such as skin cancer.
For complete information on HUMIRA, please see the official site.
Biosimilar Medications to HUMIRA
 HUMIRA costs approximately $3,100 per month ($1,000 per vial) and sales of HUMIRA worldwide are approximately $15 billion. AbbVie’s United States patent expires in December 2016 and the European patent on HUMIRA expires in most countries in 2018. In 2014, the first biosimilar of HUMIRA was released by the company Cadila Healthcare of India. Exemptia costs approximately $200 per vial. In 2016, Torrent Pharmaceuticals, an India-based company, released a second HUMIRA biosimilar medication called Adfrar at a cost of $200 per vial. Many more HUMIRA biosimilar drugs are currently in development.
HUMIRA costs approximately $3,100 per month ($1,000 per vial) and sales of HUMIRA worldwide are approximately $15 billion. AbbVie’s United States patent expires in December 2016 and the European patent on HUMIRA expires in most countries in 2018. In 2014, the first biosimilar of HUMIRA was released by the company Cadila Healthcare of India. Exemptia costs approximately $200 per vial. In 2016, Torrent Pharmaceuticals, an India-based company, released a second HUMIRA biosimilar medication called Adfrar at a cost of $200 per vial. Many more HUMIRA biosimilar drugs are currently in development.
Potential Interaction of HUMIRA and MAP
 There are no studies which specifically discuss how HUMIRA may affect Mycobacterium avium spp. paratuberculosis (MAP), however, two published articles provide preliminary data which indicate that Remicade could have a detrimental effect on the survival of MAP. These studies suggest that decreasing TNF-α levels are detrimental to intracellular MAP’s survival, possibly because other immunological pathways are activated which then suppress the growth of MAP. Since HUMIRA decreases TNF-α in a similar manner to Remicade, these studies may shed some light on the potential effect that HUMIRA could have on MAP. For a more detailed discussion of this research, see the section on Remicade.
There are no studies which specifically discuss how HUMIRA may affect Mycobacterium avium spp. paratuberculosis (MAP), however, two published articles provide preliminary data which indicate that Remicade could have a detrimental effect on the survival of MAP. These studies suggest that decreasing TNF-α levels are detrimental to intracellular MAP’s survival, possibly because other immunological pathways are activated which then suppress the growth of MAP. Since HUMIRA decreases TNF-α in a similar manner to Remicade, these studies may shed some light on the potential effect that HUMIRA could have on MAP. For a more detailed discussion of this research, see the section on Remicade.
